Abstract entitled, "Safety and Efficacy of Synchronous Ultrasound Parallel Beam Technology to Lift the Upper Lip and Improve Perioral Rhytids” selected as the highest rated Cosmetic Dermatologic Surgery Fellow Research Abstract
SAN CLEMENTE, Calif., Oct. 22, 2024 (GLOBE NEWSWIRE) -- Sofwave Medical Ltd ("the Company”) (TASE: SOFW), an emerging leader in energy-based non-invasive, aesthetic medical devices, today announced Kavita Darji, MD, FAAD, board-certified dermatologist and cosmetic dermatologic surgeon who completed her ASDS-accredited cosmetic dermatologic surgery fellowship, was selected as the winner of the 2024 Drs. Alastair and Jean D. Carruthers Award. The award is given to the highest rated Cosmetic Dermatologic Surgery Fellow Research Abstract for 2024. The award and abstract entitled, "Safety and Efficacy of Synchronous Ultrasound Parallel Beam Technology to Lift the Upper Lip and Improve Perioral Rhytids” were presented during the American Society for Dermatological Surgery's (ASDS) 2024 annual meeting which took place from October 17th through the 20th in Orlando, Florida.
Kavita Darji, MD, the study's author noted, "As a graduated ASDS accredited research fellow, I am incredibly grateful to have received this accolade from an esteemed panel of industry peers at ASDS. As people age, the production and density of elastin decreases, which can lead to wrinkles and skin laxity. The study's interim analysis highlighted the fact that in just two treatments, 93% of patients' appearance improved at the 1- and 3-month period. Moreover, the treatment effect remained in 58% of the subjects at six months after treatment. In the same way, an impressive 85% of subjects also noted a remarkable improvement in their own appearance at the 6-month period after the last treatment. The results truly place an industry spotlight on the effectiveness of Sofwave's SUPERB™ ultrasound technology - a next-generation device to easily target the mid-dermis in order to maximize new collagen production and skin elasticity.”
Louis Scafuri, Sofwave CEO, noted, "We commend Dr. Darji for her efforts to further add to Sofwave's clinical body of research. We believe that our treatments represent the future of non-invasive skin regeneration and the growth in the interest of our treatments and devices is an encouraging sign of market acceptance.”
Interim Analysis Results Highlighted in the Study
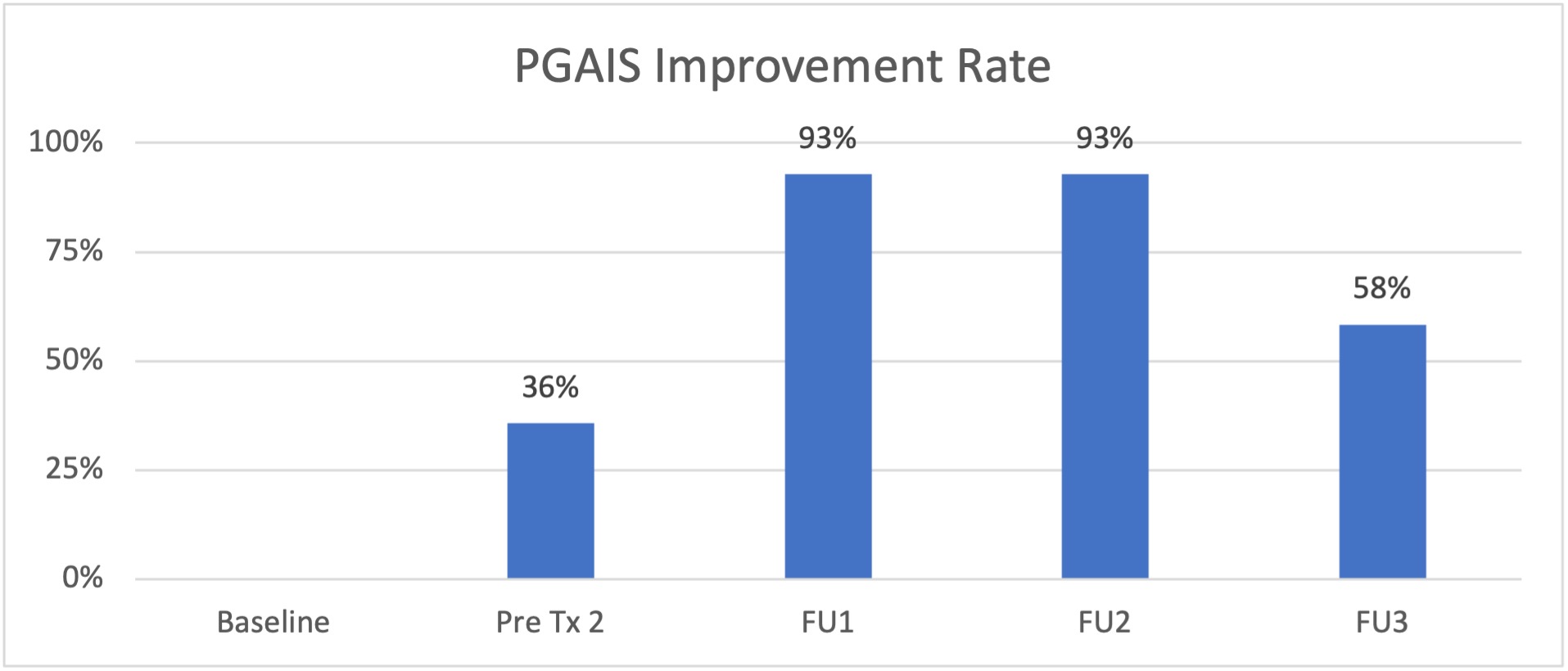
- Patients' results were measured in accordance with the Physician Global Aesthetic Improvement Scale (PGAIS).
- Following the first treatment, 36% of the subjects had an improvement according to the study investigators assessments and in accordance with the PGAIS.
- This rate increased to 93% at 1- and 3-month following the second treatment (at FU1 and FU2, respectively). The treatment effect remained at most of the subjects (58%) also six months after treatment (FU3).
The study remains ongoing.
The Drs. Alastair and Jean D. Carruthers Award is given annually and was designed by the ASDS Accreditation Work Group to stimulate interest and acknowledge cosmetic research contributions of Fellows of ASDS-accredited fellowship programs.
About Sofwave Medical
Sofwave Medical Ltd. has implemented an innovative approach to wrinkle reduction lifting and cellulite using its proprietary breakthrough technology. SUPERB™, Synchronous Ultrasound Parallel Beam technology, is FDA-cleared to improve facial lines and wrinkles, lifting the eyebrow and lifting lax submental tissue (beneath the chin), to improve the appearance of skin laxity on the upper arms, as well as the short-term improvement in the appearance of cellulite and treatment of acne scars. The company's Pure Impact™ module uses EMS technology and is cleared for muscle toning. Sofwave's products provide physicians with smart yet simple, effective, and safe aesthetic solutions for their patients. Contact: Info@sofwave.com
Investor Contact:
Brian Ritchie
LifeSci Advisors LLC
(212) 915-2578
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/722c5061-3149-46fe-b529-6d9a7ad41775



