|
Biosyngen, an innovative biotechnology company specializing in immune cell therapies, has showcased its groundbreaking technology on gene-modified, functionally enhanced tumor-infiltrating lymphocytes (TILs) derived from liver cancer biopsy samples.
BioSyngen has secured ten clinical trial approvals in China and the U.S. for its innovative fourth-generation oncology therapies. Currently, our leading pipeline product, BRG01, is in the pivotal Phase II clinical trial stage for solid tumors. Additionally, the first patients have been enrolled in the Phase I trials for our other groundbreaking therapies, BST02 and BRL03, with completion of Phase I trials anticipated later this year.
Abstract TitlePre-clinical Development of Genetically Modified Tumor-infiltrating Lymphocytes using Biopsy Samples from Liver Cancer Patients
Abstract No.
1034P
Tumor-infiltrating lymphocytes (TILs) are heterogeneous lymphocyte populations within the tumor microenvironment that contains T cells capable of recognizing tumor- or virus-associated antigens. In February of this year, the FDA approved the first TIL-based therapy used with IL-2 for advanced metastatic/recurrent melanoma. However, due to the variability in T cell infiltration between hot and cold tumors, differential abundance of antigen-specific T cells with robust immune response functionality, and the dependence of TIL anti-tumor efficacy on concomitant use of high-dose IL-2 combination therapy, the therapeutic applications of non-edited TILs are quite limited outside of melanoma.
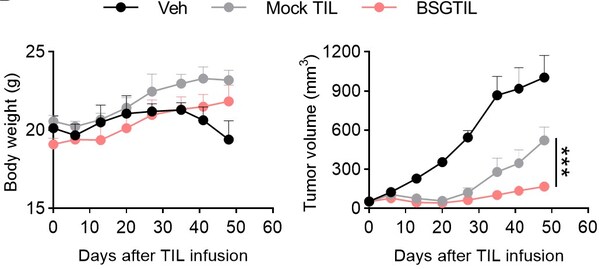
To address these challenges, Biosyngen has developed a proprietary platform for expanding TILs from biopsy samples, achieving production of 1011 cells within four weeks. Additionally, the company has established a stable gene modification platform that reprograms TIL metabolism, enhances TIL activity and sustained antitumor efficacy by expressing membrane anchor proteins. Anti-tumor efficacy has significantly enhanced (without IL-2 co-injection) and no obvious toxicity observed.
Biosyngen houses best-in-class proprietary TIL platform aiming to expand the clinical applications of TIL technology with following features:- Efficient Automated TILs Manufacturing System: Pioneering the use of tumor biopsy samples for TIL preparation with the ability to cryopreserve both tumor tissue and final products, overcoming logistical constraints.
- Effective In Vitro Gene-engineering System: Employs viral vector technology for stable gene modification, maintaining high gene expression efficiency in TILs.
- Enhanced In Vivo Expansion and Persistence: Increased proportion of central memory T cells (TCM) in the final product, leading to prolonged persistence.
- Powerful Antitumor Efficacy: Demonstrates strong tumor-killing effects without the need for concomitant use of IL-2.