|
Biosyngen, an innovative biotechnology company specializing in immune cell therapies, is presenting its groundbreaking "Conditional Activation + Armor Enhancement" T-cell technology at this year's ESMO conference.
Biosyngen has secured ten clinical trial approvals in China and the U.S. for its innovative fourth-generation oncology therapies. Currently, our leading pipeline product, BRG01, is in the pivotal Phase II clinical trial stage for solid tumors. Additionally, the first patients have been enrolled in the Phase I trials for our other groundbreaking therapies, BST02 and BRL03, with completion of Phase I trials anticipated later this year.
Abstract TitleAnti-tumor Efficacy and Safety of Conditionally Activated Armored CAR-T Cells against Gastrointestinal Tumor
Title No.
1033P
Gastrointestinal tumors have been found to exhibit significantly elevated levels of tumor-associated antigens (TAAs) compared to normal tissues, presenting potential targets for immune cell therapies. However, these TAAs are also expressed at certain levels in normal tissues, which poses a challenge for CAR-T cell therapies. Current CAR-T cells often fail to distinguish between tumor and normal tissues, leading to damage to normal organs and adverse clinical reactions. This on-target off-tumor toxicity not only raises safety concerns in clinical treatment but also limits dosage and efficacy enhancement.
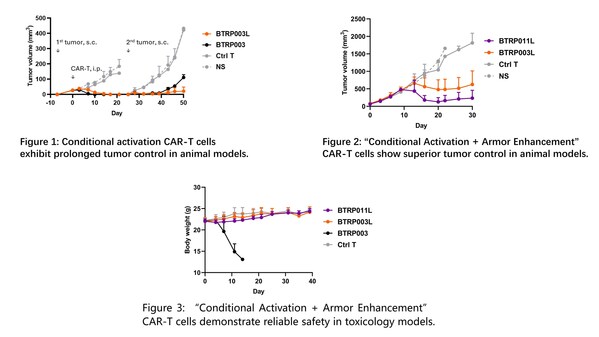
To overcome this technical challenge and balance safety with efficacy in solid tumor immune cell therapies, Biosyngen has developed the SUPER-T™ T cell safety optimization platform and created the Conditional Activation CAR-T cell BTRP003L. This technology allows CAR-T cells to activate only under hypoxic conditions typical of the tumor microenvironment (0.3% ~ 2.0% O2), while remaining inactive in normal tissues (3.4% ~ 6.8% O2). Remarkably, this approach not only regulates CAR-T cell activation levels under different conditions effectively but also enhances their antitumor capabilities (Figure 1). Further advancements include the co-expression of a TGFβRII dominant-negative mutant, resulting in the "Conditional Activation + Armor Enhancement" CAR-T cell BTRP011L, which shows improved efficacy and in vivo persistence at lower doses (Figure 2). Toxicology studies using the SUPER-T™ platform showed no weight loss or other adverse effects in mice injected with BTRP003L or BTRP011L, demonstrating robust safety (Figure 3).
About Biosyngen's Innovative Technology PlatformsIdentifier™ Platform: A drug discovery platform providing a solid foundation for the discovery of antigens, antibodies, and TCRs. With a database of hundreds of TCRs and tumor proteins, this platform supports rapid screening and optimization, identifying the most unique and suitable tumor antigens. This platform serves as a pioneer. As the ancients said, "Before troops are mobilized, provisions must be in place," which is precisely why the Identifier™ platform exists - to obtain the necessary "provisions" more rapidly.
MSE-T™ Platform: A T-cell function enhancement platform that improves the ability of immune cells to recognize tumor-specific antigens and overcome the immunosuppressive tumor microenvironment, thereby more effectively in treating difficult-to-treat solid tumors.
SUPER-T™ Platform: A T-cell safety optimization platform that enhances the safety of immunotherapy through conditional activation in the tumor microenvironment. This platform ensures maximum safety for the drug.